Home / All / Loose Lay Vinyl Flooring /
COVID -19 KN95 3D Protective Face Mask Disposable Respirator Non-Surgical FDA CE White List KN95
COVID -19 KN95 3D Protective Face Mask Disposable Respirator Non-Surgical FDA CE White List KN95
| Categories | Loose Lay Vinyl Flooring |
|---|---|
| Brand | Hanflor |
| Model | HCS 004 |
| Name | KN95 Protective Mask (FDA EUA Authorized) |
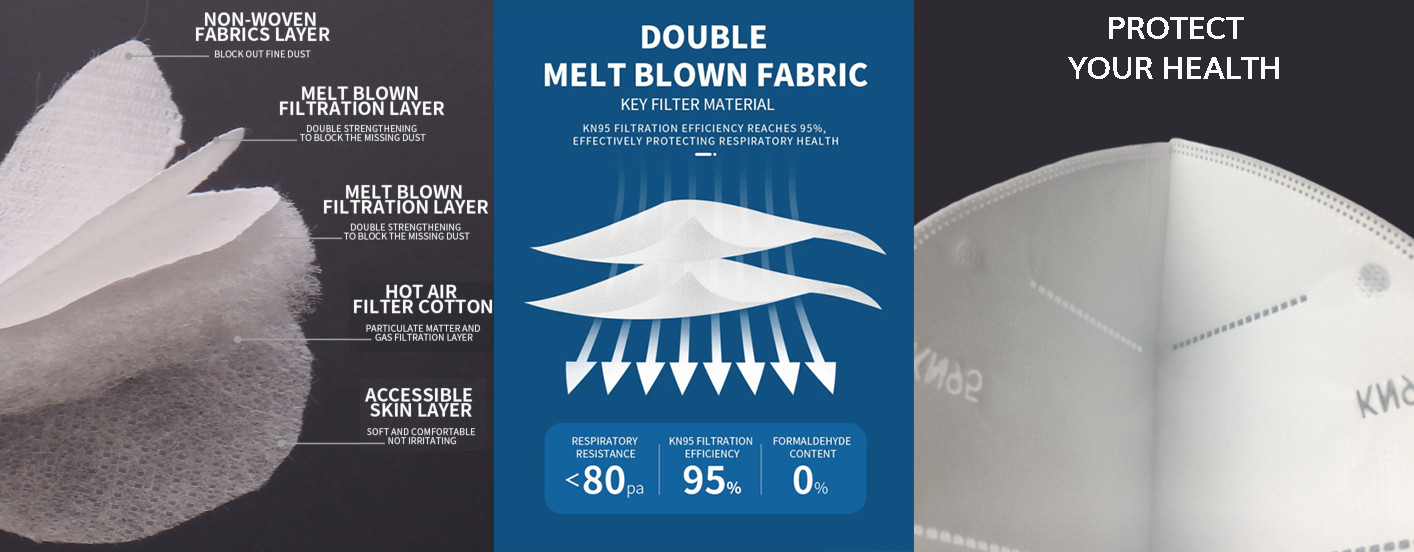
| Material | Non-Woven+Melt Blown+Hot Air Filter Cotton |
| Product Model | LSK2020-2 |
| Salty particulate filtration rate | ≥95% |
| Layer | 5 |
| Wearing Method | Ear-hook |
| Certifications | FDA(EUA),CE(SGS) |
| FOB port | SH/NB |
| Terms of Payment | T/T |
| Update Time | Apr 25,2025 |
Detail Information
INFORMATIONS
- Name
- KN95 Protective Mask (FDA EUA Authorized)
- Material
- Non-Woven Fabric / Melt Blown Filter Material / Electrostatic Heat Sealing Cotton
- Feature
- Adjustable Nose Strip(outside), Elastic Earloop
- Function
- Anti Dust / Particulate Matter / Smoke / Mist / Microorganisms / Droplets,Water Resistant
- Packing
- 1pcs/bag, 50pcs/box, 1000pcs/ctn
- Box Size
- 56cm*39*cm*50cm
- Layer
- 5
- Applications
- Healthcare / Foodservice / Industrial Safety / Laboratory / Homecare
- Standard
- GB2626- 2006
- Certifications
- FDA(EUA),CE(SGS)
PROTECTIVE MASK DETAILS


FACTORY


CERTIFICATIONS

WEARING METHOD
- 1. The nose clip part is facing up, the side without nose clip is facing the face, and the ear straps are pulled on each hand;
- 2. Face the mask against the chin first, and pull the ear-band back to the ear and hang it on the ear until it feels comfortable;
- 3. Place the fingers of both hands in the middle of the nose clip and press inwardly according to the shape of the nose bridge to conform to the shape of nose bridge;
- 4. Put your hands on your face when using, and check the tightness with your face;
FAQ
- Questions about CE Certification:
- Most of the masks on the market with CE certification are fake. Normally CE certification takes us a at least 6months time(including testing, report issue, factory facility inspection, and issue CE certificate). Due to the urgent needs of the anti-epidemic materials, EU has released the access conditions for masks. Imported masks need to have testing report following GB2626-2006 or EN149:2001+A1:2009 standard. Our masks already have GB2626-2006 and will come out with the EN149:2001+A1:2009SGS testing report in May, along with the Declaration of conformity. You can rest assured to buy.
- Questions about FDA Certification:
- The FDA do not have certificate, FDA is not a lab but a administration. they only provide medical devices registration service in the United States, so the exsiting so-called FDA certificate is fake. Due to the COVID -19 epidemic situation, FDA urgently opened a green channel to the anti-epidemic materials, till now there are 86 civil use KN95 mask suppliers who passed Chinese GB2626-2006 stardard were permitted to enter US under FDA EUA(emergency used authorized). You can rest assured to buy, there are no legal or quality risks in buying our masks.
Related Products
Please send your message to us
- Tel
- *Title
- *Content

Subscribe us here
subscription
Company
contact
- Tel:
86-0571-85265001
- Email:
- info@hanhent.com
- Address:
- Room 301-303,Building 8,No.181 Wuchang Avenue,Wuchang Street,Yuhang District,Hangzhou,China
QR code







